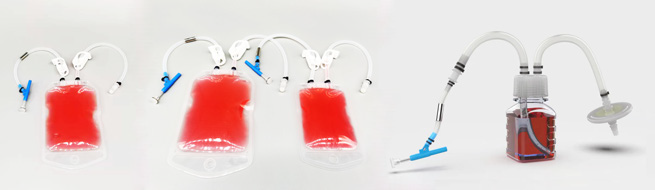
無菌サンプリングシステム
Sterile Sampling System
Product sampling is necessary and critical for cell culture and other operations in biopharmaceutical processes. Samples collected through Sampling Bags can be used to determine critical purity attributes, such as sterility, endotoxin levels, bioburden, and important cell culture parameters including metabolites, nutrient levels, pH, and osmolarity. Sterile Sampling Systems provides a pre-assembled sampling solution. It is specially designed for sampling operations at various stages of biopharmaceutical processes such as in-process monitoring of buffer storage, medium preparation, product collection and analysis. To mitigate the risk of residual contamination and ensure the safety of bio-process, the product is sterilized by irradiation prior to delivery.
Features
• Types of sampling container: bags and bottles
• Volume range: sampling bag 50 mL to 1 L, sampling bottle 20 mL to 250 mL
• 2 mm needle, covers a variety of liquid sampling needs in the entire bio-process
• The material of the liquid contact layer of both the sampling bags (ULDPE) and sampling bottles (PC) complies with bio-pharmaceutical requirements
• High transparency and excellent compatibility
• Overmolded needles and tubings for assurance of airtightness and sterility
• Adequate validation documents to ensure safety in use
• Operating temperature range: sampling bag − 80 ° C to 60 ° C, sampling bottle − 80 °C to 121 °C
• Maximum operating pressure: single-needle, single-bag products: 0.5 bar; single-needle, 5-bag products: 0.3 bar
• Customization available
Validation Documents
• 100% integrity testing
• USP<665>, Extractable testing
• USP <88> , Class VI plastics
• USP <87>, Cytotoxicity
• USP <788>, Particulate Matter in Injections
• USP<85>, Bacterial Endotoxins
• ISO 11137, Sterility testing
• ISO 10993-4, Hemolysis testing
オプションを選択
